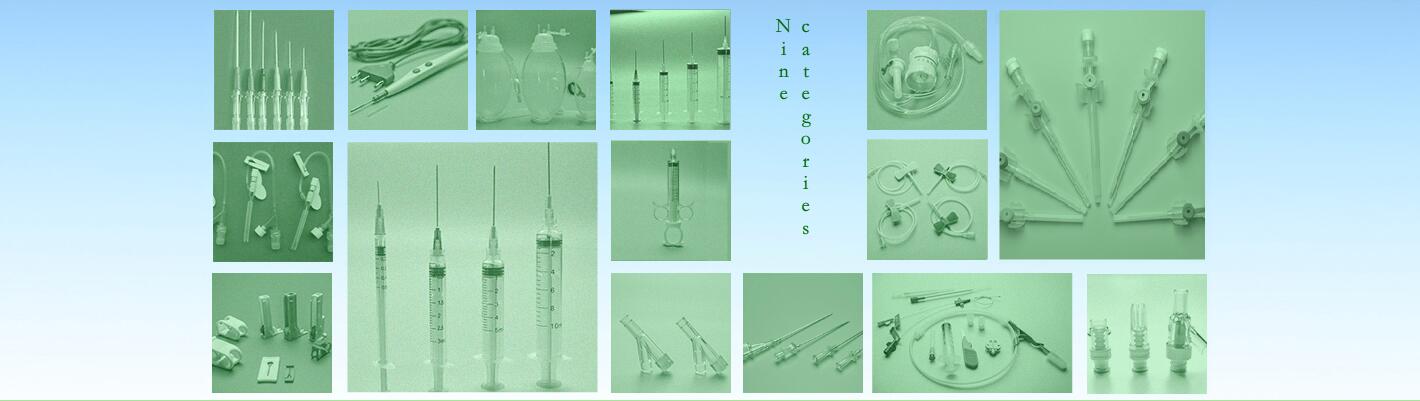
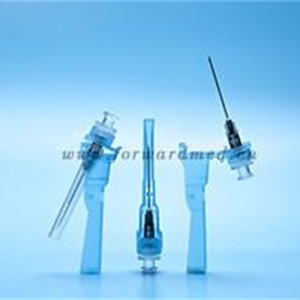
Safety hypodermic needles
1) intended use
This product is used for human body Subcutaneous, intramuscular or intravenous injection, etc.
2) main parts
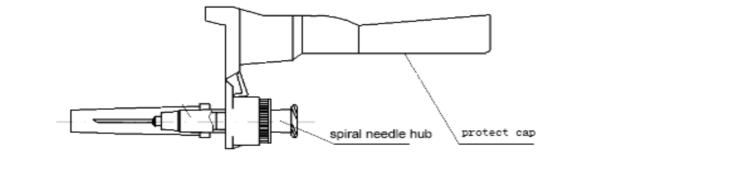
1. protect cap 2.needle tube 3.needle hub 4.plunger 5.piston 6.Plunger cap
3)Classification
ClassⅡa, according to MDD Annex IX, rule 2
4)Product Specification
Size:16G,17G,18G,19G,20G,21G,22G,23G,24G,25G,26G,27G,28G,29G,30G
5) Description of Packaging
PE/Blister individual package
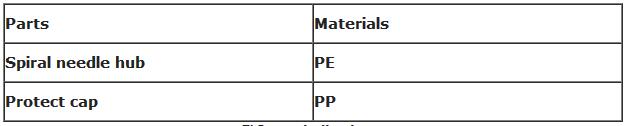
6) Bill of material
7)Contraindications:
No absolute contraindication.
8)Storage:
1. During transportation, the product shall be prevented from great pressure, direct sunlight and rain and snow;
2. The product shall be stored indoors with the humidity no more than 80%, without corrosive gas, cool, dry and well-ventilated.
9)Notice:
1、 Do not use if the package has been previously opened or damaged.
2、 Every access of the store room should keep clean and sterile.
3、 The validity sterilization period is 5 years.